 - A SKEPTIC's GUIDE
- A SKEPTIC's GUIDE  - A SKEPTIC's GUIDE
- A SKEPTIC's GUIDE Where did the energy come from? What mass got converted? To answer this question we must look at the processes involved on a sub-microscopic scale. First we must consider the natural tendency for oversized atomic nuclei to spontaneously split into smaller components.24.4 This process is known as NUCLEAR FISSION and is the energy source for all presently functioning NUCLEAR REACTORS on Earth. [Also for so-called "atomic" bombs.]24.5
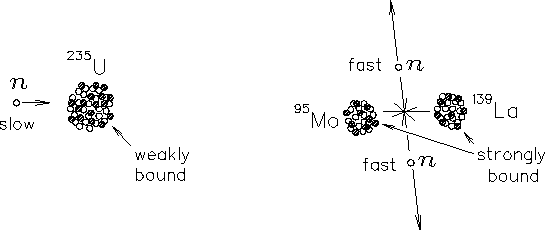 |
The basic event in the most common variety of NUCLEAR FISSION
is the spontaneous splitting of one 236U nucleus into (for example)
95Mo, 139La and two neutrons.24.6
[There are numerous other possible fission products.
This is just one case.] The fraction of the total mass
that gets converted into kinetic energy is
![]() or about a tenth of a percent.
The energy liberated in the fission of one 236U nucleus
produced in this way is 208 MeV or
or about a tenth of a percent.
The energy liberated in the fission of one 236U nucleus
produced in this way is 208 MeV or
![]() J.
That means it takes
J.
That means it takes
![]() such fissions to produce one joule
of utilizable energy. Since there are
such fissions to produce one joule
of utilizable energy. Since there are
![]() such nuclei in one gram of pure 235U metal,
such nuclei in one gram of pure 235U metal,
![]() isn't such a large number!
isn't such a large number!
What sort of control do we have over this process? To answer this question we must understand a bit more about the details of the CHAIN REACTION whereby an appreciable number of such fissions take place.
The 236U nucleus is formed by adding one neutron to a 235U
nucleus, which is found in natural uranium ore on Earth at a
concentration of about 0.72% [the rest is almost all 238U].
Now, left to its own devices (i.e., if we don't drop any slow neutrons
into it) a 235U nucleus will live for an average of
0.7038 billion years, eventually decaying spontaneously
by ![]() particle emission (not the fission reaction
that produces more neutrons!) just like its brother isotope
238U, whose lifetime is only about 6 times longer
(4.468 billion years). If the lifetimes weren't so long, there
wouldn't be any left on Earth to dig up - which might be regarded
as a good thing overall, but we have to play the hand we're dealt.
So an isolated 235U nucleus generally sits around doing nothing
and minding its own business; but when a slow neutron
comes by (picture a ball bearing slowly rattling down through
a peg board) it has a strong tendency to be captured
by the 235U nucleus to form 236U, and then the action starts.
This is also a little tricky, because if the 236U nucleus
gets a chance to settle into its ground state
(i.e., if all the jiggling and vibrating caused by absorption of
a neutron has a chance to die down) then it (the 236U nucleus)
is also quite stable [mean lifetime = 23.42 million years] and
also decays by
particle emission (not the fission reaction
that produces more neutrons!) just like its brother isotope
238U, whose lifetime is only about 6 times longer
(4.468 billion years). If the lifetimes weren't so long, there
wouldn't be any left on Earth to dig up - which might be regarded
as a good thing overall, but we have to play the hand we're dealt.
So an isolated 235U nucleus generally sits around doing nothing
and minding its own business; but when a slow neutron
comes by (picture a ball bearing slowly rattling down through
a peg board) it has a strong tendency to be captured
by the 235U nucleus to form 236U, and then the action starts.
This is also a little tricky, because if the 236U nucleus
gets a chance to settle into its ground state
(i.e., if all the jiggling and vibrating caused by absorption of
a neutron has a chance to die down) then it (the 236U nucleus)
is also quite stable [mean lifetime = 23.42 million years] and
also decays by ![]() emission (no new neutrons).
However, this is rarely the case; usually the excitations caused
by absorbing that extra neutron are too much for the excited
236U nucleus and it fissions as described earlier,
releasing several not-too-fast neutrons.
emission (no new neutrons).
However, this is rarely the case; usually the excitations caused
by absorbing that extra neutron are too much for the excited
236U nucleus and it fissions as described earlier,
releasing several not-too-fast neutrons.
What follows depends upon the neighbourbood in which the fission occurs. If the original 235U nucleus is off by itself somewhere, the two neutrons just escape, rattle around until they lose enough energy to be captured by some less unstable nuclei, and the process ends. If the fission occurs right next to some other 235U nuclei, then the outcome depends (critically!) upon the MODERATION [slowing down] of the neutrons: when they are emitted in the fission process, they are much too fast to be captured by other 235U nuclei and will just escape to bury themselves eventually in some innocuous nuclei ensewhere. If, however, we run them through some MODERATOR [slower-downer] such as graphite, heavy water (deuterium oxide, D2O) or, under extreme conditions of density and pressure, uranium metal itself, the neutrons will slow down by a sort of frictional drag until they reach the right energy to be captured efficiently by other 235U nuclei. Then we get what is known as a CHAIN REACTION. One neutron is captured by a 235U nucleus which splits up into fission products including fast neutrons, which are moderated until they can be captured by other 235U nuclei, which then split up into fission products including fast neutrons, which are . . . .
The moderation of the neutrons generates a lot of heat in the moderator (it is a sort of friction, after all) which can be used in turn to boil water to run steam turbines to generate electricity. [Or misused to make a large explosion.] A good fission REACTOR design (like the Canadian CANDU reactor) involves a moderator like heavy water (D2O) which boils away when the reactor core overheats, thus stopping the moderation and automatically shutting down the reactor. A bad design (like the Soviet or American reactors) uses MODERATOR RODS that are shoved into the core mechanically and can get stuck there if the core overheats, as happened at Three Mile Island and (much worse) at Chernobyl.24.7